Table of contents
The burden of diabetes in the world: The facts and figures
Regenerative Strategies: Pathways to Healing
Stem cell therapy and cell transplantation
Stimulating Existing Beta Cells (Proliferation)
Obstacles and The Next Horizon
Introduction
Maintenance of hyperglycemia defines diabetes mellitus as a chronic disorder of metabolism, which is now a world health burden posing varying treatment challenges.
The pathogenesis involves some form of dysfunction or death of the pancreas’s beta cells, which are endogenous insulin-producing cells.
Regeneration of these lost or injured beta cells would surely rank among the most grievous and possibly curative breakthroughs in diabetes research.
The burden of diabetes in the world: The facts and figures.
Some of the latest calls to arms for the management of the diabetes epidemic are, quite differently from historically thought, towards a cure:
Prevalence: 590 million adults are living with diabetes, according to the International Diabetes Federation (IDF).
Projections indicate that by 2050, the figure will rise to 853 million.
Deaths: Diabetes is estimated to account for some 4.2 million deaths every year worldwide.
Type 1 vs. Type 2: Of all diabetes cases, around 90% are Type 2 Diabetes (T2D), whereas 5%-10% are diagnosed as Type 1 Diabetes (T1D).
Loss of beta cells: In T1D, almost complete cessation of insulin secretion occurs from autoimmune attacks that destroy the beta cells.
In T2D, beta-cell function is progressively lost, in association with a progressive mass loss (40-60% in some reported studies), which, along with insulin resistance, results in overt diabetes.
Lately, studies suggest that overt diabetes can manifest after about a 65% loss of beta cell mass.
Thus, continued loss of functional beta cell mass continues to substantiate the case for regeneration of lost functional beta cell mass as potentially curative, at least radically disease-modifying, and not merely maintenance of blood glucose levels through insulin replacement therapy and certain classes of drugs (e.g., GLP-1 agonists and DPP-4 inhibitors).
💡Regenerative Strategies: Pathways to Healing:
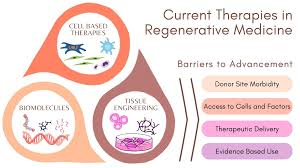
Regenerative medicine includes driving the body inside out to be able to generate its own insulin. There are three major interrelated approaches directed at generating new functional beta cells:
1. Stem cell therapy and cell transplantation
Patients will use transplants to create outside insulin-producing cells later.
- Pluripotent Stem Cells: Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) are both differentiated in the laboratory into so-called “beta-like cells” to be implanted as a replacement for lost mass.
- Clinical Advances: Involved companies ViaCyte and Vertex Pharmaceuticals are conducting clinical trials, e.g., NCT02239354 and NCT03163511, aimed at developing experimental stem-cell-derived islet progenitor cells.
In the phase 1/2 study, ViaCyte’s VC-02™ device, implanted in T1D, exhibited engraftment and maturation into insulin-releasing islet cells, with C-peptide (a marker of endogenous insulin production) becoming positive in more than 33% of subjects.
Islet transplantation is, therefore, regarded as a better surgical alternative to improve glucose control; however, it is marred by the occurrence of very rare cadaveric donation and the recipient having to be on immunosuppressant medications for life
2. Transdifferentiation
This might turn other cell types in the pancreas or in locations that are easier to access into insulin-secreting beta cells.
•Alpha-to-Beta Conversion: The pancreatic alpha cells, which are accountable for the release of glucagon, share the most important lineage with the beta cells.
Using transcription factors, such as inhibiting Arx expression, to manipulate the reprogramming of alpha cells into functional beta-like cells in animal models.
Ductal Progenitor Cells: The duct cells of the pancreas can be said to possess an attribute that is progenitor-like.
An important advancement in this area is that in the current research, the duct cells from diabetic donors were induced to adopt a beta-like cell identity in response to EZH2 inhibitors, which are FDA-approved cancer drugs like GSK126 and Tazemetostat, which enable the cells to produce and secrete insulin in response to glucose.
3. Stimulating Existing Beta Cells (Proliferation).
This would activate the remaining residual.
Understanding: Understanding the drugs that promote the activity of a crucial signaling pathway will form a universal base for all researchers working in the respective field.
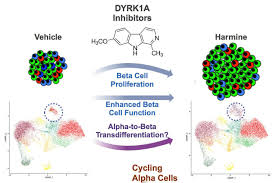
DYRK1A Inhibitors: Among the new small molecules, harmine is an example that has been found to have the ability to increase the number of beta cells by the process of inhibiting
dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 1A (DYRK1A).
GLP-1 Analogs (e.g., exenatide and liraglutide): GLP-1 Analogs have been proven to render beta cells’ life and growth even better. beta cells and encourage their within-image replication and expansion of their mass.
🚧 Obstacles and The Next Horizon
Yet, along with this set of undeniable advancements, there remain several challenges that need to be overcome in the attempt to transform beta cell regeneration into a global remedy:
- Immune Attack (T1D): The newly formed beta cells are shielded from the attack of the immune system, which T1D mounted on the original beta cells. These include:
- Encapsulation devices, such as ViaCyte’s (though hypoxia concession was a problem in earlier designs).
- Immune-evasive cells: These are designed so that the transplanted cells would be less visible or even immune-tolerant.
- Immune reset: Islet transplants combined with blood stem cell transplants to produce a “hybrid immune system,” which does not attack the new beta cells, demonstrated success in mouse models.
- Scalability & Functionality: Not trivial, high-quality, high-yield production of fully mature, glucose-responsive beta cells from stem cells.
- Safety: Unwanted growth on other tissues or off-target effects caused by these drugs should be avoided.
A huge shift in diabetes treatment from lifelong management to a cure is happening with the convergence of stem cell biology, immune engineering, and the discovery of potent regenerative molecules.

Leave a Reply